NEWS
NEWS
Why anodizing treatment can improve the corrosion-proofness of aluminum housings
17-01-2026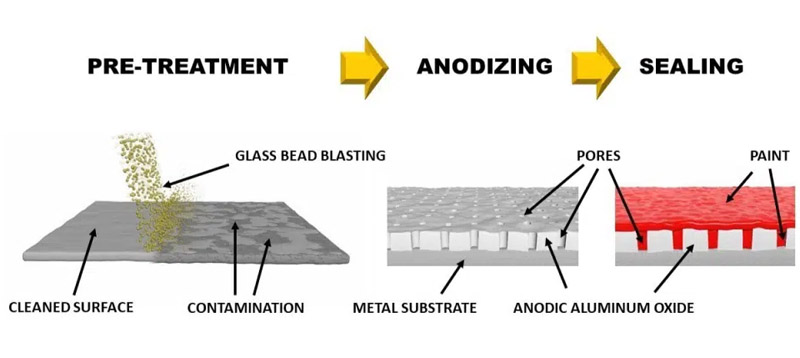 Anodizing technology is widely considered the "gold standard" for protecting aluminum housings because it doesnt just put a cover on the aluminum alloy—it transforms the surface of the aluminum alloy itself into a ceramic-like shield.
Anodizing technology is widely considered the "gold standard" for protecting aluminum housings because it doesnt just put a cover on the aluminum alloy—it transforms the surface of the aluminum alloy itself into a ceramic-like shield.While raw aluminum naturally forms a very thin oxide layer when exposed to air, this layer is too fragile for industrial or outdoor use. Anodizing improves corrosion-proofness through three primary mechanisms such as massive increase in barrier thickness, elimination of "Filiform" and pitting corrosion and sealing process.
The anodizing process adopts an electric current to "grow" the aluminum oxide layer much deeper and thicker than nature could alone. Typically, the anodized layer is 5 to 25 microns or up to 100 microns thicker, making it much harder for moisture,salt and oxygen to reach the vulnerable raw aluminum underneath.
Unlike paint or powder coating, which "sits" on top and can peel or flake, the anodized layer is molecularly bonded to the substrate. It cannot be undercut by corrosion because there is no gap between the coating and the metal.
Finally, sealing is a very important part of anodizing process. When aluminum is first anodized, the new oxide layer is actually porous , that is to day, filled with microscopic holes. To make it corrosion-proof, the aluminum housing is placed in hot deionized water or a chemical bath. This causes the aluminum oxide to hydrate and swell, physically plugging the pores.
You can imagine that anodizing process creates the the oxide layer, but Sealing "locks the joints" of the oxide layer so nothing can leak through.


